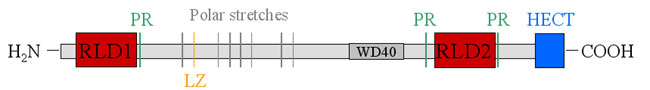
Transfection of DNA isolated from a human breast adenocarcinoma in the nude mouse tumorigenicity assay led to the identification of an oncogene termed onc H. Later on the protein was termed p532 according to its molecular weight and HERC1 according to its functions. HERC1 is consistently overexpressed in several tumor cell lines. HERC 1 consists of 4862 amino acids: |
||||||
 |
||||||
RLD1 is a GEF (guanine nucleotide exchange factor) for ARF-1, Rab3a and Rab5, all three of which are GTPases involved in cellular membrane trafficing. RLD2 specifically binds to ARF-1 in the Golgi apparatus as well as to clathrin and the molecular chaperone Hsp70. For RLD2 as yet no GEF activity has been shown. |
||||||